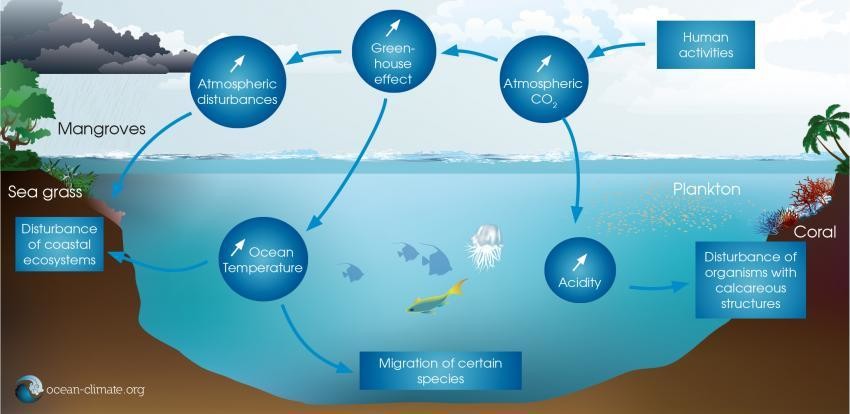
The ocean is not only affected by climate change; it may also play a role in reversing it.
Because of its capacity to avoid land use, direct ocean carbon capture (DOC) is an emerging form of negative emissions technology that has advantages over its on-land cousin, direct air capture. DOC can also be used in conjunction with offshore wind and offshore carbon dioxide storage.
Katherine Hornbostel is an assistant professor of mechanical engineering and materials science at the University of Pittsburgh Swanson School of Engineering. She has been actively engaging with Pitt’s Department of Chemical and Petroleum Engineering Assistant Professor Tagbo Niepa to create unique ocean carbon capture methods.
The team submitted two related publications, “Demonstration of direct ocean carbon capture using encapsulated solvents” and “Demonstration of direct ocean carbon capture using hollow fiber membrane contactors,” in the Chemical Engineering Journal. These two publications show, experimentally and computationally, how two types of membrane contactors, encapsulated solvents and hollow fiber membrane contactors, can remove CO2 from the ocean.
“Membrane contactors are just what they sound like,” Hornbostel explained. “They’re membranes that allow two fluids to interact with one another.” In this example, we have ocean water on one side and a solvent on the other.”
The researchers put two types of membrane contactors to the test: hollow fiber and encapsulated solvents. The form of the two technologies is the most noticeable distinction between them. Encapsulated solvents resemble caviar, while hollow fiber membrane contactors resemble straws. Otherwise, they function identically.

“The idea with both is to get a really high surface area of contact between the seawater and the solvent,” Hornbostel added. “The more surface area you have, the better the carbon dioxide removal rate.”
Swinging the seawater Carbon dioxide will desire to flow through the membrane towards the solvent, which is generated from a sodium solution that reacts with CO2. When seawater interacts with the solvent, carbon dioxide reacts and separates from the seawater. The solution must then be re-circulated to make the process more cost effective, something the team is presently working on.
RELATED: The Fight To Expose Corporations’ True Climate Impact
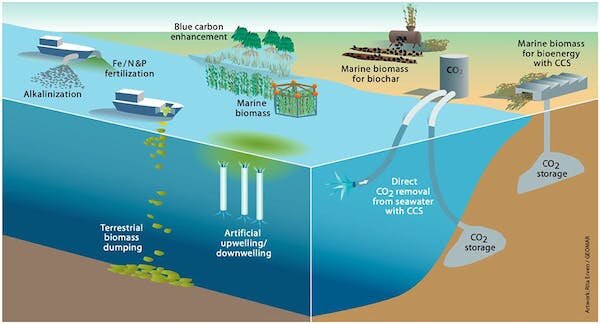
“Theoretically, we could significantly lower the price if we could swing the pH of the seawater side,” Hornbostel explained. “Carbon dioxide is not typically available in seawater at its baseline level of pH, so you have to swing the pH lower in the seawater to make it more acidic, and then more carbon dioxide bubbles off.”
Hornbostel’s team is presently studying methods for swinging seawater pH with membrane surface treatments, as well as the possibility of combining direct ocean capture with desalination to reduce system costs.
Download The Radiant App To Start Watching!
Web: Watch Now
LGTV™: Download
ROKU™: Download
XBox™: Download
Samsung TV™: Download
Amazon Fire TV™: Download
Android TV™: Download

